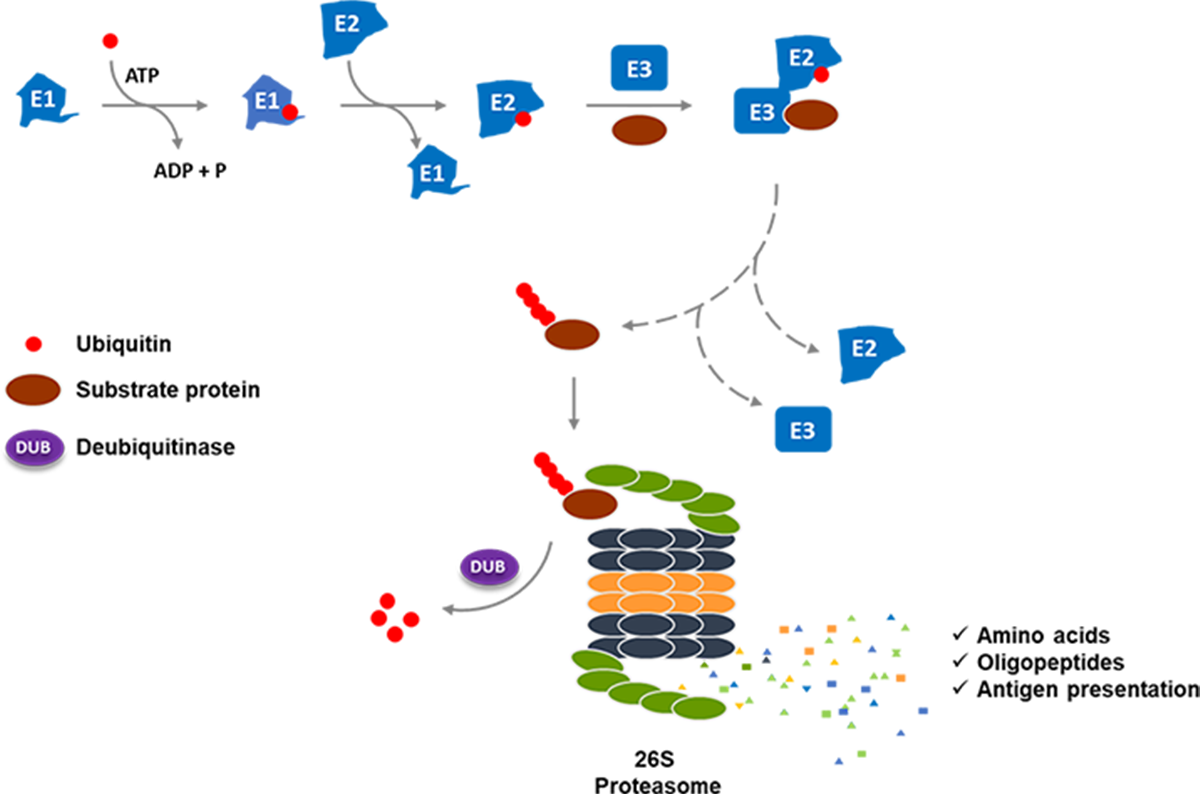
Recently, cereblon (CRBN), a 442-amino acid protein, has been identified as a substrate receptor of the CRBN-CRL4 E3 ligase complex (CRL4CRBN) targeted by a class of immunomodulatory small molecule drugs, including thalidomide and its derivatives lenalidomide and pomalidomide.
A growing body of evidence has shown that substrate binding specificity of the CRL4CRBN E3 ligase complex can be modulated by thalidomide and its analogs, leading to recruitment and accommodation of new, non-intrinsic substrates for ubiquitination and degradation. Four neo-substrates of the CRL4CRBN E3 ligase complex have been well characterized: Ikaros (IKZF1) and Aiolos (IKZF3), two important members of the Ikaros family of zinc-finger transcription factors responsible for the maturation of B cells, CK1α and GSPT1.
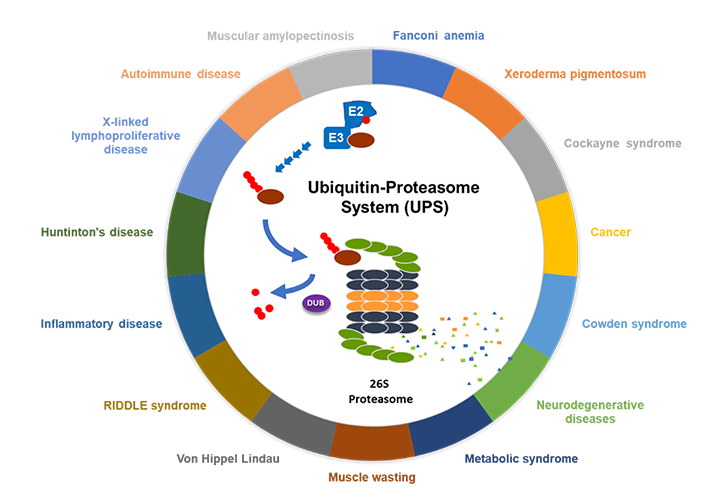
Modulating substrate specificity of CRL4CRBN E3 ligase complex via novel small molecules to destroy disease-associated proteins, particularly those that lack discrete catalytic domains amenable to small-molecule inhibition, has tremendous potential and a bright future in the drug development field.
Over the past several years, Kangpu Biopharmaceuticals has established a broad and solid pipeline covering novel generation of small molecular entities that modulate CRL4CRBN E3 ligase complex with promising potential for the treatment of solid tumors, hematological malignancies, auto-immune diseases as well as inflammatory disorders.
References
- 1. Ito T. et al. Science Mar 2010
- 2. Zhu Y. et al. Blood Nov 2011
- 3. Lu G. et al. Science Jan 2014
- 4. Fischer E. et al. Nature Aug 2014
- 5. Krönke J. et al. Science Jan 2014; Nature Jul 2015
- 6. Petzold G. et al. Nature Apr 2016
- 7. Matyskiela M. et al. Nature Jul 2016
- 8. Sievers Q. et al. Science Nov 2018