Kangpu Biopharmaceuticals Received IND Approval from NMPA for KPG-818 to Treat Relapsed/Refractory Multiple Myeloma
Feb 25, 2025
Hefei, China, February 25, 2025 -- Kangpu Biopharmaceuticals, Ltd. (“Kangpu”) today announced that the Company has received IND approval from the CDE (Center for Drug Evaluation) of China National Medical Products Administration (NMPA) for KPG-818 for the treatment of relapsed/refractory multiple myeloma (RRMM).
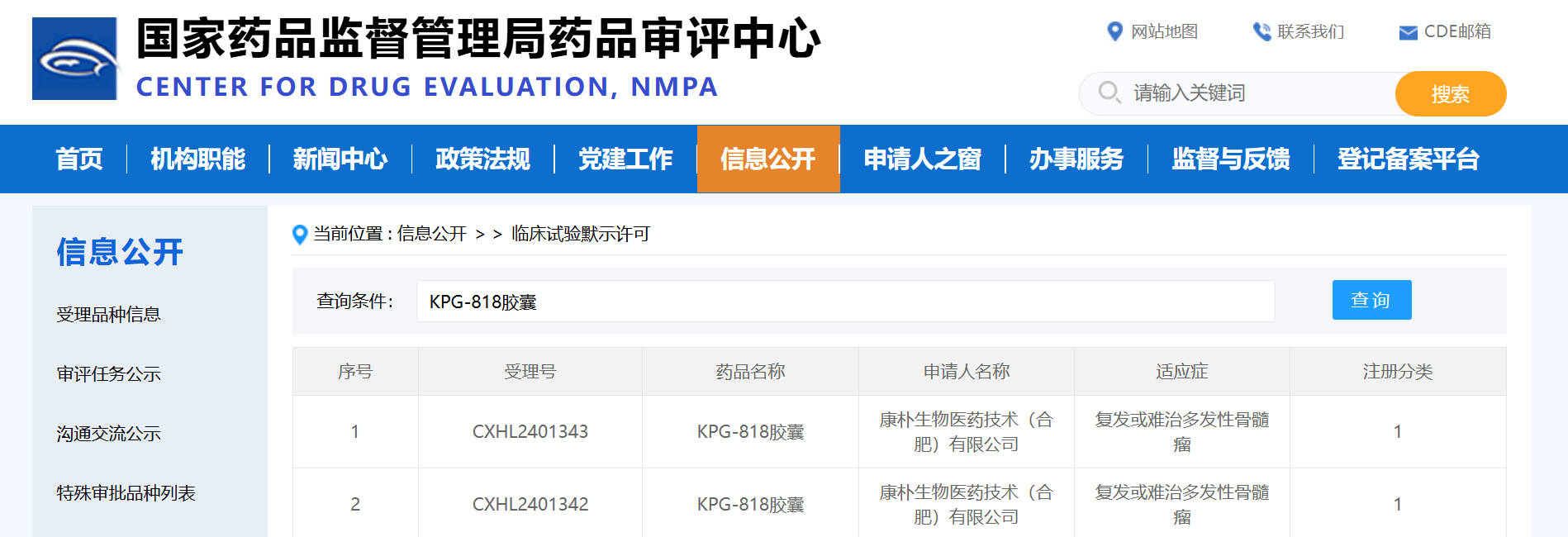
KPG-818 is a novel oral molecular glue modulator of the E3 ubiquitin ligase complex CRL4-CRBN. It demonstrated high cereblon (CRBN) binding affinity and potent degradation of Aiolos (IKZF3) and Ikaros (IKZF1), two members of the Ikaros family of zinc-finger transcription factors associated with B-cell development. KPG-818 possesses immunomodulatory, anti-angiogenic, and anti-tumor effects.
The Phase I clinical trial of KPG-818 in the United States for the treatment of various hematological tumors has been completed. Preliminary results indicate that in RRMM patients who have previously received two immunomodulatory drugs (lenalidomide and pomalidomide), at least one proteasome inhibitor (bortezomib, ixazomib, or carfilzomib), and a CD38 monoclonal antibody (daratumumab or isatuximab), KPG-818 demonstrated good safety, tolerability, pharmacokinetic characteristics, and encouraging therapeutic effects.










